Research
VIVI Cap Stabilizes Insulin in Extreme Temperature Conditions
Andreas Pfützner 1, Wolfgang Reeh2, Ron Nagar3, Dirk Rose4, 1Pfützner Science & Health Institute, Diabetes Center and Practice, Mainz, Germany; 2Diabetes Care Centre, Oppenheim, Germany 3TempraMed Inc., Tel Aviv, Israel; 4Flymed Aviation Centre, Frankfurt, Germany
Introduction
For long-term storage, Insulin is to be kept at 4-8° C (~ 39°F to 47°F) until use and once opened, is supposed to be stable for up to 30 days at room temperature. Extreme cold or heat lead to insulin degradation in a very short time with loss of its glucose-lowering efficacy. VIVI Cap is a calorimetry-based portable storage device, which fit all existing disposable pens It works without external power supply requirements based on space-derived vacuum insulation and additional heat consumption by phase-change material. It is designed to keep the insulin cool within safe temperatures below 29°C/84.2°F for a minimum of 12 hours even in a constant environmental temperature of 37.8°C/100°F
Methods
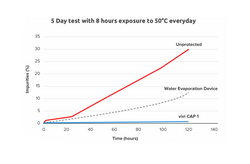
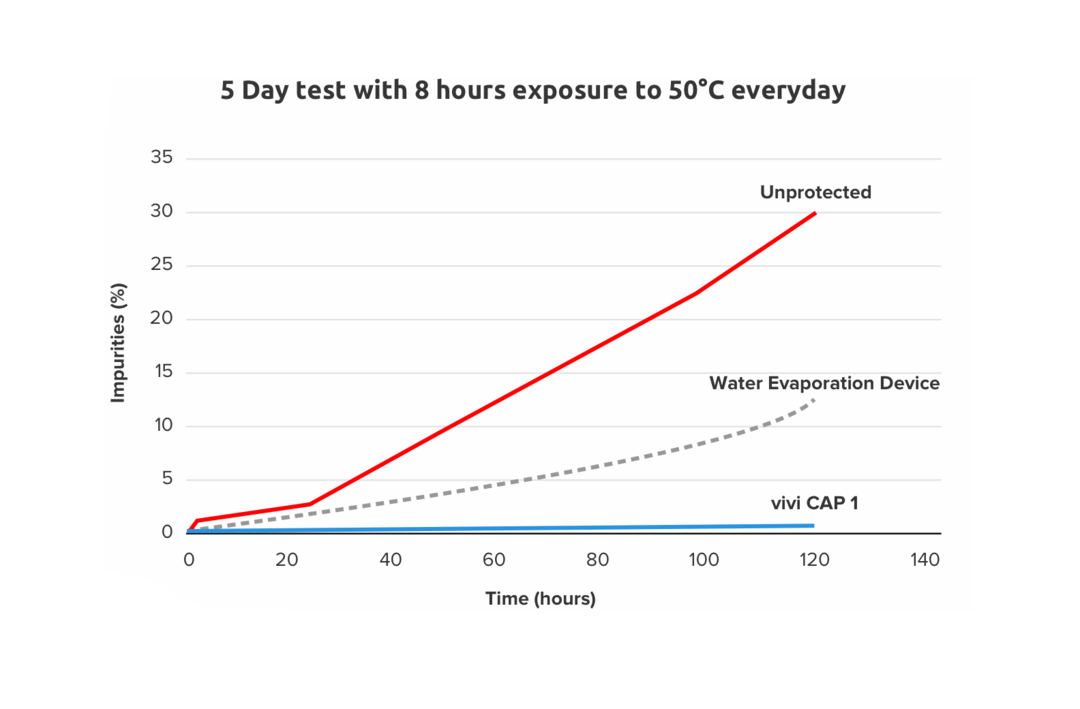
The purpose of this experiment was to evaluate the performance of the VIVI Cap 1 device, comparing it to an insulin pen without any protection, and to an insulin pen, which employs commonly used water evaporation based device for temperature protection. Three disposable insulin pens were kept for one week under extreme temperature conditions for 5 days (each day: 8 h at 50 °C and 16 h at 22 °C) either without protection, in a frio device (freshly prepared each day) and in the VIVI Cap. Samples were taken every day and insulin degradation was determined in accordance with EU pharmacopoea by appropriate HPLC methods for insulin aspart. High (HMW) and low molecular weight (LMW) degradation molecules. Each experiment was performed in triplicate.
Results
Insulin aspart without protection was shown to have more than 2 % impurities already after one day , while it took 2 days for the pens in the Frio device. The pens with VIVI Cap did not reach this level of impurities during the experiment (0.5 % after 5 days). High molecular weight products > 1 % occurred after two days without protection, three days with Frio, but no HMW products were seen in the pens protected with VIVI Cap.